
The valency (or oxidation state) of sulphur in SO2 is 4+. if we write 16 electrons of sulphur,they can filled as 2,8,6 which shows that there are 6 electrons in outermost shell of sulphur so simply we can say its valency is 6. Valence electrons are the outermost electrons which, therefore, are located on the highest energy levels or these are the electrons available for chemical bonding. Where are the valence electrons located in Sulphur? Here, the charge of Sulfur is+6 (CaSO4=Ca+2 S+6 O -8) SO4 >-2 anion as in CaSO4 (The mineral Anhydrite). H2S gas : Here the charge os sulfur is -2. Sulfur with electron configuration of 2, 8, 6, means that it could either gain TWO electrons and the charge will be -2, or LOSE six electrons and the charge will be +6. What is the charge of sulfur with two electrons? The key difference between valency and charge is that valency indicates the ability of a chemical element to combine with another chemical element, whereas charge indicates the number of electrons gained or removed by a chemical element. The net charge on an ion is represented by a superscript +, 2+, and 3+ mean a net charge resulting from the loss of one, two, or three electrons, respectively. An element’s placement on the periodic table indicates whether its chemical charge is negative or positive. A chemical charge can be found by using the periodic table.
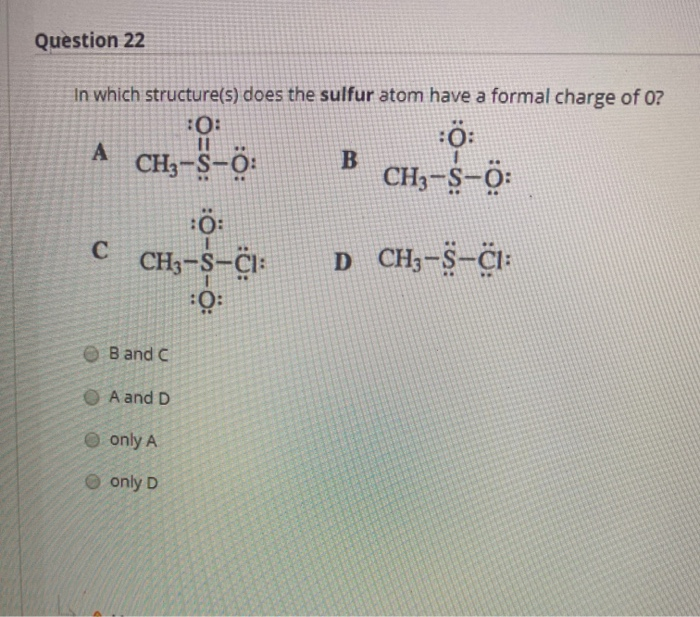
Therefore, sulfur has 6 valence electrons.Ĭharge number or valence of an ion is the coefficient that, when multiplied by the elementary charge, gives the ion’s charge. What is the charge on its ions, and is the charge positive or negative? The charge is negative, since sulfur is a non-metal.

Sulfur is in group 6 of the periodic table.
#Sulfur charge how to
5 How to find the formal charge of SO3?.4 What is the charge of sulfur with two electrons?.


 0 kommentar(er)
0 kommentar(er)
